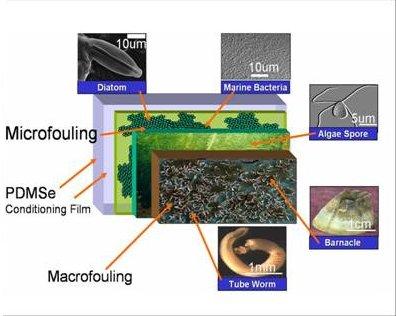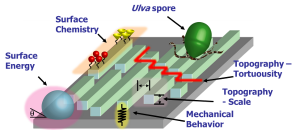
AntifoulingMarine organisms, such as algae spores and barnacles, settle and adhere to the hulls of ships causing a reduction in ship efficiency. This type of biofouling incurs large functional and monetary costs to both military and commercial vessels such as reduced ship speed due to drag, increased fuel consumption, cost of dry-docking for cleaning, and loss of hull strength from corrosion. Paints containing tributyltin (TBT) and copper have been successful at combating this problem. However, due to their toxicity, the use of these paints are not environmentally friendly and restrictions have been put on their use worldwide by 2008 [1]. A non-toxic substitute is therefore desired that reduces settlement and enhances relaese of marine biofouling organisms. Bioadhesion is a comlex process that involves living organisms and cells probing and attaching to surfaces. Surface chemistry, topography, and bulk properties of the substrate all affect the degree of settlement and strength of biological adhesion [2,3,4]. The ability to tailor a surface to control bioadhesion would have implications in applications as diverse as ultrafiltration, power plant intake pipes, and biomaterials. Our research approach involves the investigation of bioadhesion using model surfaces. The surface designs we create and study enable the examination of the complex interactions that occur with surface energy, surface chemistry, surface topography and mechanics. We are studying the bioadhesion processes of complex organisms such as the motile Ulva zoospores of common algae and Balanus barnacles. We measure the settlement behavior and adhesive strength of algae spores. In addition, we are evaluatins the probing behavior and settlement of barnacle cyprids and tubeworm larvae.
Presented here are the advances in controlling bioadhesion accomplished with microtopography. Our newest biomimetic design, which we have named Sharklet AFTM, are inspired by the structure of shark scales [5]. We have successfully demonstrated for the first time a significant reduction in settlement of the Ulva algae spores with a non-toxic polymeric coating in laboratory assays. Preliminary results with barnacles and tubeworms show evidence of an equivalent reduction in settlement with topography. We are currently applying these findings towards the design of organism-specific Sharklet AFTM surfaces.
All microtopographical surfaces were fabricated at the University of Florida. Master silicon molds of each unique surface are created using standard photolithographic techniques followed by deep reactive etching to a desired detph. Molds can then be replicated in a variety of elastomers and hydrogels. Silastic T2 (Dow Corning) polydimethylsiloxane elastomer (PDMSe) was chosen for this application due to its non-toxic nature in the marine environment and ability to replicate high fidelity microtopgraphic features.
Ulva algae assays were performed at the University of Birmingham according to established protcols [6,7]. Briefly, microscope slides coated with PDMSe topographic features were soaked in artificial seawater within individual compartments of quadriperm dishes for 1 hour. The water was then drained and 10mL of spore suspension (1.5×106 spores/mL) was added. The dishes were placed in darkness for 60 min. The slides were rinsed in artificial seawater to remove unsettled spores. Three replicates were fixed with 2.5% glutaraldehyde and spore counts were measured using a Zeiss Kontron 3000 image analysis system attached to an epifluorescence microscope and video camera. Spore counts are obtained from thirty areas on each replicate.
Balanus barnacle assays were performed at California Polytechnic State University. Three replicates of each sample were initially soaked in aged seawater for six days, replacing the water every 72 hours. The settlement assay consists of placing a 0.4mL drop of seawater containing 20-40 barnacle cyprid larvae on the experimental coatings. The larvae are allowed to settle for 72 hours, or until the settlement rate on a glass control surface reaches 50%. At this time, the number of barnacles that have settled on each surface are counted. Settlement rates on the experimental coatings are compared with the rates of the controls.
Ulva spore settlement density on the Sharklet AFTM topography was reduced an average of 76% (67% to 86% range) relative to a smooth PDMSe surface in 6 independently evaluated assays. The Sharklet AFTM geometric dimensions are designed an order of magnitude smaller than that of actual shark scales. These dimensions were specifically chosen as they restrict the Ulva spore body (4 – 6 microns) from entering the spaces between features. Previous studies with channels have identified these critical dimensions around which the Sharklet AFTM geometry is based [6,7]. A proposed mechanism that defines the inhibition of the settlement is based upon the stress produced on the ion channels of the cellular membrane caused by the gradient topography.
Preliminary results for barnacle settlement on topographical channels indicate that the critical dimensions for barnacle larvae are between 20 and 200 microns for feature width/spacing and feature depths greater than 20 microns. To contrast, algae spore critical dimensions are approximately 2 microns. For 20 micron width channels at a depth of 20 microns, barnacle settlement was reduced by approximately 65%.
The success of the Sharklet AFTM design with repelling spores has propelled our research to search for next generation topographical architectures. We will be testing a host of new Sharklet AFTM designs to increase our understanding of the settlement behavior of the Ulva spore. We will continue testing the settlement and adhesion of Balanus barnacles and move into other marine fouling organisms such as Hydroides tubeworms. We have preliminary data suggesting the specific critical dimensions to decrease barnacles settlement and are currently designing and fabricating barnacle-specific Sharklet AFTM surfaces. Hierarchical topographical surfaces are being fabricated that can encompass more than one type of topography of various size scales to affect the settlement and release characteristics of more than one organism simultaneously.
This work was funded by a grant from the Office of Naval Research (Contract No. N00014-02-1-0325).
Selected references
|
 Figure 1: Submarine fouled by algae.
Figure 2: Hierarchy of marine fouling.
Figure 3: Bioadhesion model that considers surface energy, surface chemisty, topography, and mechanics.
Figure 4: Sharklet AFTM topography created on the surface of a silicone elastomer film imaged by SEM.
Figure 5: Scanning electron micrographs showing: (A) the anti-algae Sharklet AFTM topography and (B) the anti-barnacle 20μm wide channels and (C) combined hierarchical topography for multiple organism settlement inhibition.
Figure 6: Light micrographs (100X) of algae spores (Ulva) settled on (A) Smooth PDMSe surface and {B} Sharklet AFTM topography. Bar graph indicates spore settlement density. (error bars=95% CI)
Figure 7: Performance history of Sharklet AFTM topography for Ulva spore settlement in terms of percet reduction compared to a smooth PDMSe surface.
Figure 8: Barnacle settlement preference on topographical channels compared to smooth PDMSe and glass controls (error bars=std. error) |